Structure, bonding and properties of matter
Structure, bonding and properties of matter
Level: AS Levels, A Level, GCSE – Exam Boards: Edexcel, AQA, OCR, WJEC, IB, Eduqas – Chemistry Revision Notes
Structure, bonding and properties of matter
Exam boards:
Chemical bonds
Atoms need a full outer shell to be stable. They will form chemical bonds in order to become more stable.
There are three types of chemical bond:
| Ionic
|
Covalent | Metallic | |
| Between | Metals and non-metals | Non-metals and non-metals | Metallic elements and alloys |
| Particles | Oppositely charged ions | Atoms that share electrons | Positive ions with outer electrons that can move |
| Example | NaCl | F2 | Al |
Forming ions
An ion is a charged particle.
An atom must lose or gain an electron to become charged.
- Metals lose electrons to become positively charged (e.g. groups 1 and 2).
- Non-metals gain electrons to become negatively charged (e.g. groups 6 and 7).
- Noble gases have stable atoms (full outer shells). They do not lose or gain electrons.
| Group | Electrons lost/gained | Ion formed | Example |
| 1
|
Lose 1 electron | + 1 | K K+ + e– |
| 2
|
Lose 2 electrons | + 2 | Ca Ca+ + 2e– |
| 6
|
Gain 2 electrons | -2 | O + 2e– O2- |
| 7
|
Gain 1 electron | -1 | Cl + e– Cl– |
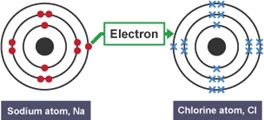
Ionic Bonding
This is when a metal atom transfers electrons to a non-metal atom in order for both atoms to become stable. The metal atom becomes a positive ion and the non-metal atom becomes negative ion.
The electrostatic attraction between the positive and negative ion is an ionic bond.
e.g. NaCl
(bbc bitesize)
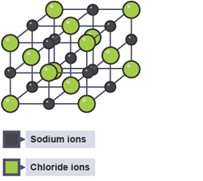
Ionic compounds from giant lattices
A lattice is a regular repeating structure.
- There are millions of strong electrostatic (between positive and negative ions) attractions in all directions.
- The lattice is 3D.
- This structure means that most ionic substances are crystal-like.
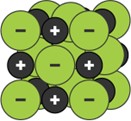
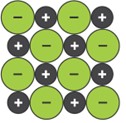
The lattice structure of an ionic compound can be shown using different models.
3D Ball and stick model 3D Space filling model 2D Space filling model
(bbc bitesize)
Properties of ionic compounds
- High melting points as lots of energy is needed to overcome the electrostatic attractions.
- High boiling points.
- Cannot conduct electricity as a solid as ions cannot move.
- Can conduct electricity when in liquid or molten form as ions are free to move and carry electrical current.
Covalent Bonding
This is when a pair of electrons is shared between two non-metals in order to give both atoms a full outer shell.
(bbc bitesize)
Simple covalent molecules
A simple covalent molecule contains only a few atoms.
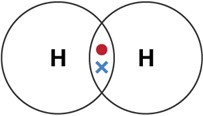
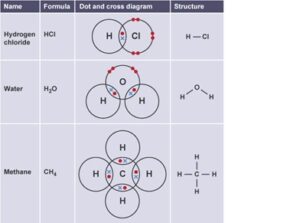
Covalent bonding can be modelled using dot cross diagrams.
- Each dot or cross represents an electron.
- The circles represent the electron shells.
- Only the outer electrons need to be shown.
- Circles must overlap where there is a covalent bond.
(bbc bitesize)
Simple molecules have weak forces of attraction between them. These are called intermolecular forces. These are the forces that need to be broken in order to melt or boil the substance.
The bigger the molecule the greater the number of intermolecular forces there are and so the higher the melting and boiling point.
Multiple bonds
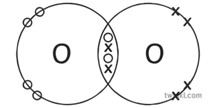
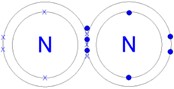
One pair of electrons between atoms is a single covalent bond. Two pairs of electrons represent a double bond (e.g. O2) and three pairs of electrons is a triple bond (e.g. N2).
(gcsescience.com)
Structural formulae
This type of diagram can represent a molecule with covalent bonding. A line represents the bond and the element symbol represents the atom. e.g. H2
Giant Covalent Structures
Giant covalent structures have many atoms joined together by covalent bonds.
Comparing giant covalent structures
| Structure | Bonding | Properties |
| Diamond
(twinkle) |
Each Carbon is bonded to four other carbon atoms
(all outer electrons involved in bonding)
|
· Very Hard
· Very Strong · High melting and boiling point · Does not conduct electricity (no free electrons) |
| Silicon Dioxide
|
Each silicon is bonded to two oxygens | · High melting and boiling point
|
| Graphite
|
Made up of layers of Carbon atoms held together by weak intermolecular forces
Each Carbon is bonded to three other carbon atoms
This leaves one free delocalised electron per carbon atom that is able to carry electric charge
|
· High melting point
· Conducts electricity
|
| Graphene
|
One layer of graphite (2D)
Each Carbon is bonded to three other carbon atoms
|
· Conducts electricity
· Extremely strong · Elastic
|
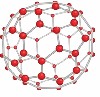
Fullerenes
Fullerenes are molecules of carbon atoms with hollow shapes such as tubes or balls. The carbon atoms are arranged into hexagons or rings of 5 or 7 carbons.
Nanoparticles
Nanoparticles are particles that are sized between 1-100nm.
1nm= 1 x 109 metres
Nanoparticles have a high surface area to volume ratio (SA:V). The higher to ratio, the greater the proportion of particles exposed at the surface.
If the side length of the cube decreases by a factor of 10, the SA:V ratio increases by 10.
Applications:
- self-cleaning glass
- sunscreens
- face creams and cosmetics
- drug delivery
- antimicrobial coatings
- catalysts
Possible risks:
- The large surface area means they are explosive.
- Breathing in the tiny particles could damage the lungs.
- Once inside the body they could enter the bloodstream.
Carbon Nanotubes
Nanotubes are tiny cylindrical fullerenes.
They are much longer than they are wide. They can conduct electricity and so can be used in electronics and nanotechnology.
They can help strengthen materials without adding too much weight.
Polymers
Polymers are covalently bonded long chain molecules.
They are made up of smaller repeating molecules called monomers.
e.g. ethene molecules make poly(ethene).
The chains are held together by intermolecular forces. Longer polymer chains have stronger intermolecular forces (forces of attraction) that shorter ones.
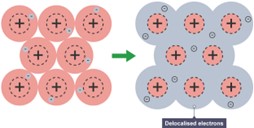
Metallic Bonding
Metallic bonding occurs between metals only. The positive metal ions are arranged in closely packed layers and are surrounded by a sea of delocalised electrons.
There is a strong electrostatic attraction between the positive metal ion and the negative electrons. This is what holds the atoms together.
Properties:
- High melting and boiling points due to the strong electrostatic attraction.
- Conducts electricity due to the delocalised electrons.
- Malleable (as the layers of atoms can slide over one another easily).
- Ductile (as the layers of atoms can slide over one another easily).
Metals are often mixed with other metals to make alloys .
The different sized atoms in the layers of atoms prevents the layers from sliding over one another. This makes the alloys harder or more difficult to bend and shape.
Questions
Question 1:
Describe what happens when a Lithium atom reacts with a chlorine atom. Answer in terms of electrons.
Answer:
The lithium atom loses one electron and the chlorine (atom) gains one electron to form positive and negative ions.
Question 2:
Graphite and fullerenes are forms of carbon. Graphite is soft and is a good conductor of electricity. Explain why graphite has these properties. Answer in terms of structure and bonding.
Answer:
Each carbon atom forms three covalent bonds forming layers of hexagonal rings. It is soft because layers can slide over each other. It can conduct electricity because delocalised electrons are free to move and carry charge.