Organic Chemistry
Organic Chemistry
Level: AS Levels, A Level, GCSE – Exam Boards: Edexcel, AQA, OCR, WJEC, IB, Eduqas – Chemistry Revision Notes
Crude Oil and Hydrocarbons
Hydrocarbons
A hydrocarbon is a molecule that is made up of hydrogen and carbon atoms only.
Crude Oil
- Crude oil is a mixture of hydrocarbons.
- It is a fossil fuel, so it is non-renewable.
- It is found in rocks and is the remains of plankton and ancient biomass compressed in mud over millions of years.
- Crude oil is mostly made up of a mixture of alkanes
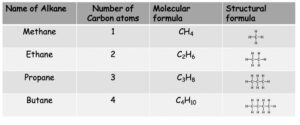
Alkanes
Alkanes are a type of hydrocarbon with the general formula CnH2n+2. They form a homologous series where they all share the general formula and have similar chemical properties.
Alkanes:
- Have single bonds between the atoms. Each carbon atom forms four bonds.
- Are called saturated compounds-there are no more bonds available to bond to other atoms.
- Have similar chemical properties but different physical properties.
- The longer the carbon chain, the higher the boiling point.
The first four alkanes
Note: You can also write the formulae of alkanes by showing the atoms bonded to each carbon atom. e.g. Butane can also be written as CH3CH2CH2CH3
Combustion
Complete combustion
Complete combustion occurs when a fuel burns in plenty of oxygen to produce carbon dioxide and water. Alkanes can be burnt using oxygen in the air.
e.g. Methane + Oxygen Carbon dioxide + water
CH4. + 2O2 CO2 + 2H2O
Incomplete combustion
Incomplete combustion occurs when a fuel burns but there is limited oxygen. The products are water, poisonous carbon monoxide and carbon.
More energy is released during complete combustion than incomplete combustion.
Fractional distillation
Figure 1: Source KS4 BBC bitesize.
- Fractional distillation is the process of separating the mixture of hydrocarbons in crude oil.
- The alkanes are separated according to their boiling points and chain lengths.
- Short chain alkanes have lower boiling points due to the molecules being held together by weak intermolecular forces.
- Long chain alkanes have higher boiling points due to the molecules being held together by stronger intermolecular forces.
- Each part of the oil is called a
Separating fractions
- Oil is heated and pumped into the bottom of a tall tower called a fractionating column, where it vaporises.
- The column is very hot at the bottom but much cooler at the top. As the vaporized oil rises, it cools and condenses.
- Heavy fractions (containing large molecules) have a high boiling point and condense near the bottom of the column.
- Lighter fractions (containing small molecules) have a lower boiling point and condense further up the column.
Note: Liquids boil at their boiling points and the hot gases condense back to liquids at the same temperature.
Properties of hydrocarbons
Fractional distillation produces many fuels with different properties depending on their chain-length. These properties will influence how they are used as fuels.
Figure 2: Table comparing the properties of short and long-chain hydrocarbons.
Cracking
Shorter chain alkanes are more in demand than long chains due to their properties. The solution to the problem of supply and demand is cracking.
Cracking is when long-chain hydrocarbons are heated and broken down into shorter chain alkanes and alkenes. It is an example of thermal decomposition.
It is carried out with:
- vapours being passed over a hot catalyst at 550oC in catalytic cracking.
- or mixing the vapours with steam in steam cracking.
Testing for products of cracking
The products of cracking include shorter alkanes and another type of hydrocarbon called an alkene.
Alkenes contain a double bond between the carbon atoms and are unsaturated as the double bond can be broken and other atoms can be added to the molecule.
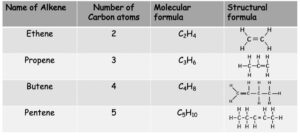
Alkenes
Alkenes have the general formula CnH2n
Note: You can also write the formulae of alkenes by showing the atoms bonded to each carbon atom. e.g. Butene can also be written as CH2CHCH2CH3
Bromine water is used to test for alkenes. It is orange in colour.
- If an alkene is added, then the bromine water will change from orange to colourless. (The double bond is broken and bromine is added onto the molecule.)
- If an alkane is added, then the bromine water will remain orange.
Reactions of alkenes
Alkenes also undergo combustion reactions. They tend tend to undergo incomplete combustion and produce a smoky flame.
Incomplete combustion of ethene:
C2H4. + 2O2 ——-> 2CO + 2H2O
Addition reactions
A functional group is the atom or the group of atoms by which the characteristic reaction of the organic compound is determined.
- Alkenes contain the functional group C=C.
- The double bond between the carbon atoms is able to undergo an addition reaction. This means that the double bond can break and can accept another molecule.
e.g. bromine will add across the double bond of ethene to produce dibromoethane (BrCH2–CH2Br)
- Alkenes react with hydrogen in a reaction called hydrogenation. The reaction requires a catalyst, where the hydrogen molecule will add across the double bond to form an alkane.
- Alkenes can also react with water to produce an alcohol. This is called a hydration reaction. The reaction requires a high temperature (300 ̊C) and a catalyst.
- Alkenes can react with halogens in a halogenation reaction in which an alkyl halide is produced.
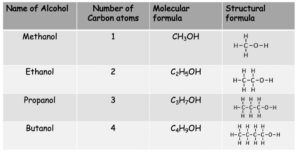
Alcohols
Alcohols contain the functional group -OH.
Making alcohol
Ethanol can be made through a process called fermentation. This uses yeast to turn glucose into alcohol in an anaerobic process (occurs without oxygen).
Fermentation requires:
- sugars from plants
- water
- enzymes which come from using yeast. (The enzymes are biological catalysts)
- warm temperatures (25-40oC)
If the temperature is too cold, then the enzymes become inactive and cannot function.
If the temperature is too hot, the enzymes are denatured and fermentation will not occur.
The ethanol produced is dilute and needs to be distilled to produce ethanol that is almost pure.
Reactions of alcohols
- Sodium will react with alcohols to produce hydrogen.
Sodium + ethanol —–> sodium ethoxide + hydrogen
2Na + 2C2H5OH —–> 2C2H5ONa + H2
- Ethanol will burn with plenty of oxygen in a combustion reaction to produce carbon dioxide and water.
C2H5OH + 3O2 ——-> 2CO2 + 3H2O
- Alcohols can be oxidised to produce carboxylic acids using an oxidising agent (e.g. potassium dichromate(VI)).
RCH2-OH ——> RCOOH + H2O
Carboxylic acids
Vinegar (ethanoic acid) is an example of a carboxylic acid. Carboxylic acids contain carbon, hydrogen and oxygen atoms and behave as other acids do.
- They dissolve in water to produce acidic solutions
- They react with metal carbonates to produce salts (called ethanoates) and carbon dioxide.
- They will turn universal indicator yellow or orange as they are weak acids (they do not ionise completely in water).
Carboxylic acids contain the functional group -COOH.
Esters
Alcohols will react with carboxylic acids in the presence of an acid catalyst to produce esters. This is called a condensation reaction.
Esters smell fruity and are often used in perfumes.
Figure 2 shows an ester link.
Alcohol + carboxylic acid ester + water
Figure 2: Source KS4 BBC bitesize
Naming esters
- Change the name of the alcohol to end in –yl. This part comes first.
- Change the name of the carboxylic acid acid to end in –oate. This part comes second.
e.g. Methanol + ethanoic acid ⇌ methyl ethanoate + water
Hydrolysis of esters
An ester can be broken up by heating the ester with an alkali such as sodium hydroxide. This is an example of a hydrolysis reaction as a water molecule is added and breaks up the structure.
Addition polymerisation
A polymer is a very long chain molecule made up of repeating molecules called monomers. Poly means ‘many’.
Alkenes can be used as monomers and many alkene molecules can be joined (added) together to form a long chain. This is addition polymerisation.
e.g. lots of ethene molecules can be added together to form poly(ethene).
The double bond in ethene is broken and forms a single bond instead.
Figure 3: Poly(ethene) is formed from many ethene molecules.
Source KS4 BBC bitesize
There are normally thousands of ethene molecules joined up together, so the letter ‘n’ is used to represent the large number of molecules.
Figure 4: Source KS4 BBC bitesize
Condensation polymerisation
Condensation polymerisation uses two monomers that have different functional groups. The two molecules react together to form a new functional group plus a small molecule such as water.
An example of this process is forming an ester. The -OH group from an alcohol can react with the -COOH group from a carboxylic acid to form an ester plus water.
alcohol + carboxylic acid —-> ester + water
R-OH + R’COOH —-> R’COOH + H2O
Polyesters
A polyester is formed when the monomers have two of the same functional groups on one monomer. A condensation reaction is used to create a long polymer chain.
e.g. ethanediol has two OH groups and hexanedioic acid has two COOH groups
n HO-CH2– CH2-OH + n HOOC- CH2- CH2- CH2– CH2-COOH ————>
[-CH2– CH2-OOC- CH2– CH2– CH2– CH2-COO-]n + 2n H2O
Amino acids
Amino acids are the molecules that make up peptides and proteins. Proteins are essential for growth and repair. There are 20 different types of amino that create the proteins that are found within our cells.
An amino acid contains the amine group (-NH2) and the carboxyl group (-COOH). They bond together through condensation reactions to produce polypeptides.
Figure 5: Source KS4 BBC bitesize
Many proteins act as enzymes (biological catalysts) and are essential to biochemical functions.
DNA and biological polymers
There are many naturally occurring polymers in nature. Starch and cellulose are polymers of sugars. Proteins are polymers of amino acids.
DNA is also a naturally occurring polymer. It is made up of a double helix which is two polymer chains that are twisted to form the ladder shape.
The monomers are called nucleotides. They are made up of three parts:
- A sugar molecule
- A phosphate group
- A base
The sugar molecule and the phosphate group are always the same. They form the backbone of the polymer.
The four nucleotides (bases) in DNA are:
- A (adenine),
- G (guanine),
- C (cytosine)
- T (thyroxine).
The nucleotide sequence codes for genes. A and T always pair together, C and G always pair together. It is the order of bases within the polymer chain that encodes the genetic information.
Questions
- Write a balanced symbol equation for the complete combustion of propane
- Explain how you would test to detect the difference between butane and butene
- What are the conditions required for making ethanol by fermentation?
Answers
- C3H8 + O2 3CO2 + 4H2O
- Pass both substances through bromine water. Butane will not cause a colour change. Butene will cause a colour change from orange/brown to colourless.
- Sugar, yeast and water are needed. Yeast is needed to provide the enzymes for the reaction to take place. The temperature must be between 25-40o It must be carried out in anaerobic conditions.