Chemical Changes
Chemical Changes
Level: AS Levels, A Level, GCSE – Exam Boards: Edexcel, AQA, OCR, WJEC, IB, Eduqas – Chemistry Revision Notes
Chemical Changes
The reactivity series
The reactivity series is a list of metals in order of reactivity. Observations of the metals with substances such as oxygen, water have been used to place the metals.
The most reactive metals are at the top and the least reactive at the bottom
Hydrogen and carbon are also placed in the reactivity series although they are non-metals.
Why are some metals more reactive than others?
Different metals have different tendencies to lose their outer electrons and form positive ions.
- The further away from the nucleus the outer electron is, the less attractive force there is, so the electron is lost more easily.
- The more electron shells there are, the further away the outer electron will be from the nucleus.
- Sodium forms ions much more easily than lithium or copper.
A more reactive metals will displace a less reactive metal from a compound of the less reactive metal. This is called a displacement reaction.
e.g. Iron sulphate + magnesium —–> magnesium sulphate + iron
Reactions of metals
Reactions of metals with water
When metals react with water, a metal hydroxide and hydrogen gas is produced.
Metal + Water —–> Metal Hydroxide + Hydrogen
Potassium + water —–> Potassium Hydroxide + hydrogen
2K + 2H2O —–> 2KOH + H2
The more reactive the metal, the faster the reaction.
Reactions of metals with oxygen
Metals react with oxygen to form metal oxides.
e.g. Iron + oxygen —–> iron oxide
When a substance gains oxygen, it is called an oxidation reaction.
Reactions of metals with dilute acid
Metals react with acids to produce a salt and hydrogen.
Sodium + hydrochloric acid —–> sodium chloride + hydrogen
2Na + 2HCl —–> 2NaCl + H2
Metals that are below hydrogen in the reactivity series do not react with dilute acids.
Extraction of metals
Metals that are very unreactive such as gold can be found naturally in the Earth. These metals are called native metals.
Most other metals are found as compounds because they react with other elements (ores). There are two main methods to extract metals:
- Electrolysis: Used for metals more reactive than carbon
- Reduction: Used for metals less reactive than carbon
Reduction using carbon
A reduction reaction is when oxygen is removed from a compound.
Displacement reactions can be used to remove oxygen in order to extract a metal from an ore.
e.g. To extract zinc there are two stages:
- The zinc ore (ZnS) is converted to Zinc Oxide
- The zinc oxide is reduced to zinc
Zinc oxide + carbon —–> zinc + carbon dioxide
2ZnO + C —–> 2Zn + CO2
Reduction of Zinc half equation:
2Zn2+ + 4e– —–> 2Zn
Reactions of acids
When an acid reacts with an alkali, a salt and water are produced. This is called a neutralisation reaction.
acid + alkali —–> salt + water
hydrochloric acid + sodium hydroxide —–> sodium chloride + water
HCl + NaOH —–> NaCl + H2O
Reactions of a base and an acid
Metal compounds (bases) will react with acids to produce a salt
- Metal oxide + acid —–> salt + water
- Metal hydroxide + acid —–> salt + water
- Metal carbonate + acid —–> salt + water + carbon dioxide
Naming salts
In order to name salts:
- The first part of the name comes from the metal in the compound
- The second part of the name comes from the acid
| Acid | Salt name | Ion |
| hydrochloric | chloride | Cl– |
| nitric | nitrate | NO3– |
| sulphuric | sulphate | SO42- |
e.g. Sodium hydroxide + Hydrochloric acid + —–> Sodium Chloride + water
Sodium Carbonate + Hydrochloric acid + —–> Sodium Chloride + water + Carbon dioxide
Making salts
Reacting metals with acid
- Excess metal is added to the acid.
- The solution is filtered into a crystallising dish to remove unreacted metal.
- The solution is concentrated by evaporation (normally by heating).
- The remaining concentrated solution is left to evaporate at room temperature and crystallise
Making soluble salts
e.g. Copper oxide + Sulphuric acid —–> copper sulphate + water
- Make a saturated solution by adding an excess of the base into the acid until no more will dissolve. The acid can be heated to allow more base to react.
Image: AQA required practical booklet
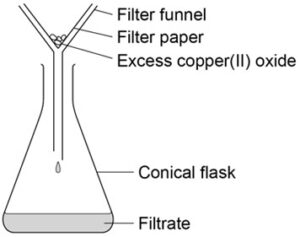
2. Filter the solution using filter paper and a funnel to remove the excess unreacted solid base
Image: AQA required practical booklet
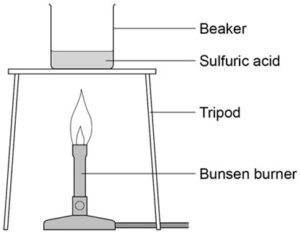
3. Half fill a beaker with water and set this over a Bunsen burner to heat the water. Place an evaporating dish on top of the beaker.
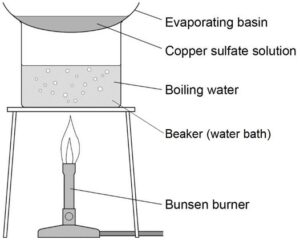
4. Add the solution to the evaporating basin and heat until concentrated and crystals begin to form.
Image: AQA required practical booklet
5. Once cooled, pour the remaining liquid into a crystallising dish and leave to crystallise
6. Remove the crystals with a spatula and pat dry between filter paper.
Ionic Equations
Ionic equations show what happens to the electrons in a reaction. The more reactive a metal is, the easier it loses its electrons.
In a displacement reaction the ions of the more reactive metal are ‘forced’ onto the ions of a less reactive element.
Magnesium + Zinc Sulphate —–> Magnesium Sulphate + Zinc
Mg + ZnSO4 —–> MgSO4 + Zn
- Split the compounds into their ions.
Mg + Zn2+ SO4 2- —–> Mg2+ SO42- + Zn
2. Remove the spectator ions (the ones that do not lose or gain electrons in the reaction.)
Mg + Zn2+ —–> Mg2+ + Zn
3. Write half equations-one for each metal to show which electron transfer is happening.
Mg – 2e– —–> Mg2+
Zn2+ + 2e– —–> Zn (The zinc ion gains electrons to make zinc atom)
Redox reactions
A redox reaction is when oxidation and reduction takes place at the same time.
Hydrochloric acid + calcium —–> calcium chloride + hydrogen
2HCl + Ca —–> CaCl2. + H2
An ionic equation is used to show this process.
- Oxidation is the loss of electrons (OIL)
- Reduction is the gain of electrons (RIG)
2H+ + Ca —–> Ca2+ + H2
The ionic equation can be split into two half equations.
Oxidation-Calcium loses two electrons
Ca —–> Ca2+ + 2e-
Reduction-Hydrogen gains two electrons
2H+ + 2e- —–> H2
The pH scale
Image: BBC bitesize
An acid is a substance that produces H+ ions in aqueous solution.
An alkali is a substance that produces OH– ions in aqueous solution.
Universal indicator (UI) can be added to solutions. The colour changes depending on the number of H+ ions in the solution.
- The higher the concentration of H+ ions the lower the pH
- The lower the concentration of H+ ions the higher the pH
Neutralisation
Hydrogen ions from an acid react with hydroxide ions from an alkali to produce water.
H+ (aq) + OH-(aq) —–> H2O (l)
Strong and weak acids
- A concentrated acid contains a large number of acid particles. The concentration of an acid tells you how many moles of acid there are in a dm3.
- A dilute acid contains a smaller number of acid particles.
- A strong acid completely dissociates in a solution.
Hydrochloric acid dissociates in solution to form hydrogen and chloride ions.
HCl —–> H+ + Cl-
- A weak acid only partially dissociates in solution.
Ethanoic acid partially dissociates to form a hydrogen and CH3COO–.
The double arrow symbol shows that the reaction is reversible.
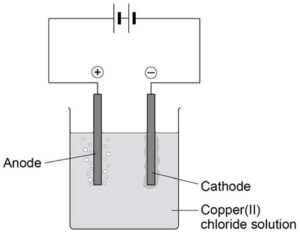
Electrolysis
Electrolysis is the splitting up of an ionic substance using electricity.
- Electrode: conducting rod
- Anode: positive electrode
- Anion: negative ion
- Cathode: negative electrode
- Cation: positive ion
- Electrolyte: ionic solution that is being split up
- Inert: unreactive
Image: AQA required practical booklet
- An electrical circuit is set up.
- The anode and the cathode are placed in the
- The electrodes are inert (so that they do not react in the reaction) and are often made from graphite or platinum.
Opposites attract
The positively charged ions are attracted toward the cathode. The negatively charged ions are attracted towards the anode.
When ions reach the electrodes, the charges are lost by either losing or gaining electrons and they become elements.
Electrolysis of molten and aqueous compounds
Electrolysis can only take place if the ionic compound is molten or in aqueous solution as the ions are free to move and carry the charge.
Molten Compounds
Oxidation is the loss of electrons and reduction is the gaining of electrons. OIL RIG.
A molten compound is one that has been melted and so it is a liquid.
e.g. Molten lead bromide
PbBr2 is made up of the ions Pb2+ and Br–
The lead ions are attracted toward the negative cathode at the same time as the bromide ions are attracted toward the positive anode.
At the cathode: lead gains two electrons to become a lead atom (REDUCTION)
Pb2+ + 2e– —–> Pb
At the anode :each bromine ion loses an electrode to become a Br atom. The two atoms then covalently bond to form a Br2 molecule. (OXIDATION)
2Br– —–> Br2 + 2e–
Extraction of Aluminium
Aluminium is manufactured by electrolysis. Aluminium oxide has a very high melting point, therefore melting it would use large amounts of energy and increase the cost of the process. Molten cryolite is added to aluminium oxide instead to lower the melting point and thus reduce the cost.
Aqueous (ionic) Solutions
Rules for ionic solutions
In an aqueous solution there are four types of ions. The positive and negative ions from the ionic compound and H+ and OH– from water molecules.
H2O —–> H+ + OH-
This means there are four ions in solution.
The least reactive ions will be released as their elements (discharged) and the more reactive ions stay in solution.
- If the metal is MORE reactive than hydrogen, then hydrogen will be produced at the cathode.
- If the metal is LESS reaction than hydrogen, then the metal is produced at the cathode.
- If the negative ion is a HALOGEN, then the halogen is produced at the anode.
- If the negative ion is not a halogen (e.g. SO42- , CO32- or NO3-) then oxygen is produced at the anode.
NaCl (aq) solution
At the cathode: : 2H+ + 2e– —–> H2
At the anode: 2Cl– —–> 2e– + Cl2
Titrations
Titration is a method which allows the accurate addition of a volume of one solution of a known concentration to a known volume of another solution of unknown concentration until the reaction reaches neutralisation, the end point is indicated by a colour change.
The results from a titration experiment can be used to calculate the concentration of a solution or the volume of solution required to neutralise an acid or alkali.
Titration Method
- Use a pipette to measure 25cm3 of alkali and pour into a conical flask.
- Add several drops of phenolphthalein indicator to the alkali solution.
- Swirl the flask, the mixture will turn pink.
- Place the conical flask on a white tile.
- Fill the burette with acid to the 0 line.
- Place the burette over the conical flask.
- Slowly open the tap so the acid flows into the conical flask. Swirl the flask until the indicator changes from pink to colourless.
- Continue to add the acid drop by drop until the indicator is permanently colourless in the flask.
- Record the total volume of acid added to the alkali.
- Repeat the experiment two more times and work out an average.
Using the results
- concentration = moles ÷ volume (dm3)
- number of moles (of solute) = concentration (mol/dm3) x volume (dm3)
- volume (dm3) = moles ÷ concentration (mol/dm3)
Example
10cm3 of 2.0 mol/dm3 sulfuric acid reacted with 25cm3 of sodium hydroxide. What was the concentration of sodium hydroxide?
NaOH + H2SO4 —–> Na2SO4 + H2O
Step 1: Balance the equation
2NaOH + H2SO4 —–> Na2SO4 + 2H2O
Step 2: Convert cm3 to dm3 (divide by 1000)
- 10 ÷ 1000 =. 01 dm3 of sulphuric acid
- 25÷ 1000 = 025 dm3 of sodium hydroxide
Step 3: Work out the number of moles of acid.
number of moles = concentration × volume
moles= 2 x 0.01 =0.02 moles of acid
Step 4: Using the equation work out the number of moles of alkali
The equation shows that 2 moles of sodium hydroxide react with 1 mole of acid.
So the ratio of acid: alkali is 1: 2
So the number of moles of alkali: 0.02 ÷ 2 = 0.01 moles
Step 5: Work out the concentration of the alkali.
concentration = number of moles ÷ volume
concentration = 0.01 ÷ 0.025 = 0.8 mol/dm3
Questions
- What is a displacement reaction?
- How can displacement be used to extract a metal from an ore?
- What is electrolysis? Which metals can it be used to extract?
- What would be the products of the electrolysis of molten zinc chloride?
- What would be the products of the electrolysis of sodium chloride solution?
Answers
- A reaction in which a more reactive metal takes the place of a less reactive metal in a compound
- Displacement reactions can be used to remove oxygen in a reduction reaction in order to extract a metal from an ore. g. 2ZnO + C 2Zn + CO2. Carbon removes the oxygen from the zinc oxide as it is more reactive than zinc.
- Electrolysis is the splitting up of an ionic substance using It is used for metals more reactive than carbon.
- Zinc metal and chlorine gas
- Hydrogen gas and Chlorine gas.